Featured
Kymriah Car T
Kymriah ist die einzige zugelassene CAR-T-Zell-Therapie die die 4-1BB kostimulatorischen Domäne verwendet welche für die volle Wirkungsentfaltung der Therapie die Verbesserung der zellulären Ausbreitung und die dauerhafte Persistenz der krebsbekämpfenden Zellen unerlässlich ist. 25 Zeilen Übersicht behandelnder CAR-T Zentren in Deutschland.
 Novartis Fills Manufacturing Gap For Car T Therapy Kymriah With First Asian Production Facility Fiercepharma
Novartis Fills Manufacturing Gap For Car T Therapy Kymriah With First Asian Production Facility Fiercepharma
The 4-1BB costimulatory domain is responsible for enhancing the expansion and persistence of KYMRIAH.

Kymriah car t. Kymriah das in Zusammenarbeit mit der University of Pennsylvania Penn entwickelt wurde zählt zu den individuellen Therapieverfahren. In August 2017 the first CAR chimeric antigen receptor-T cell therapy became available in the USA in the form of Kymriah tisagenlecleucel. This usually takes less than 1 hour.
CAR T-Zellen wie Tisagenlecleucel Kymriah. Kymriah is the first-ever FDA-approved CAR-T cell therapy and the first-ever CAR-T to be approved in two distinct indications. Tisagenlecleucel wird als CAR-T-Zell-Therapeutikum.
Kymriah was granted FDA approval for children and young adults up to the age of 25 years who have acute lymphocytic B-cell leukemia that is therapy-refractory or has relapsed at least twice. You should plan to stay within 2 hours of the location where you received your treatment for at least 4 weeks after. Serious side effects occur in most patients.
Specifically designed to enhance early T-cell expansion and long-term endurance of CAR-T cells23 Demonstrated induction of central memory T-cell differentiation for enduring protection and immunosurveillance in vitro3 May help KYMRIAH CAR-T cells evade some of the bodys native immunosuppressive stimuli4. 342 Patienten wurden seit EU-Zulassung der CAR-T-Therapien Yescarta und Kymriah im Herbst 2018 mit einem dieser beiden Produkte in Deutschland bisher behandelt Quelle. When your body is ready your health care provider will give you KYMRIAH through a tube intravenous catheter in your vein.
KYMRIAH tisagenlecleucel is indicated for the treatment of paediatric and young adult patients up to and including 25 years of age with B-cell acute lymphoblastic leukaemia ALL that is refractory in relapse post-transplant or in second or later relapse. The most common serious side effects are cytokine release syndrome a potentially life-threatening condition that can cause fever vomiting. Die Behandlung mit der Kymriah-CAR-T-Zelltherapie erfolgt ein einziges Mal und kommt in Deutschland nur für eine sehr kleine Patientenpopulation von wenigen Hundert Patienten mit fortgeschrittenen.
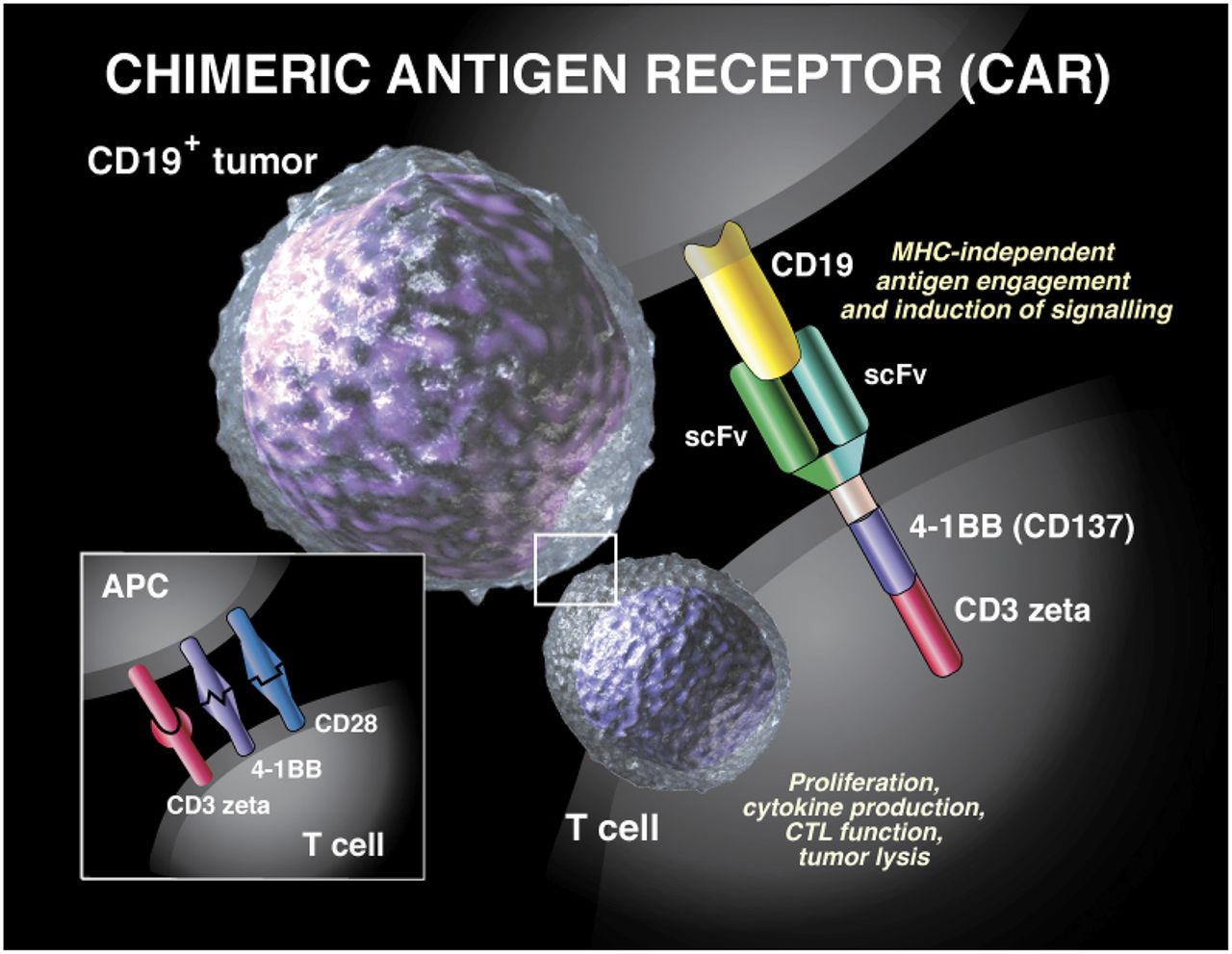
KYMRIAH is an individualized therapy that reprograms a patients own T cells with a chimeric antigen receptor CAR containing a 4-1BB costimulatory domain. KYMRIAH is provided as a single-dose for infusion containing a suspension of chimeric antigen receptor CAR-positive viable T cells. Zwei NGOs haben sich gegen das Patent von Kymriah gewehrt es sei kein Arzneimittel sondern eine medizinische Dienstleistung.
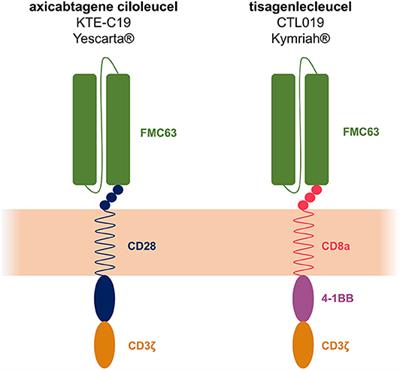
Before you get KYMRIAH your health care provider may give you chemotherapy for a few days to prepare your body. Dies erfolgt zunächst durch eine Abnahme von patienteneigenen T-Zellen die mit einem Rezeptor ausgestattet Chimärer Antigenrezeptor CAR und über 2 -4 Wochen im Labor vermehrt werden. The co-stimulation zone of Kymriah uses 4-1BB while the co-stimulation zone of Yescartas CAR structure uses CD28.
Pediatric and Young Adult 25 years old RR Acute Lymphoblastic Leukemia ALL Get the information youll need about KYMRIAH CAR-T cell therapy so you can have a confident conversation with the doctor about your childs ALL. Last year the FDA approved two CAR-T products to be marketed namely Kymriah of Novartis and Yescarta of Gilead. Diese Zulassung basiert auf dem Review der beiden einzigen globalen klinischen CAR-T.
Images throughout do not depict actual KYMRIAH. Administer 06 to 60 x 10 8 CAR-positive. Tisagenlecleucel sold under the brand name Kymriah is a medication for the treatment of B-cell acute lymphoblastic leukemia ALL which uses the bodys own T cells to fight cancer adoptive cell transfer.
It is a one-time treatment designed to empower patients immune systems to fight their cancer. Diese patienteneigenen CAR-T Zellen können jetzt die Krebszellen nach einem Schlüssel-Schloss-Prinzip erkennen. Im August 2017 wurde mit Kymriah Tisagenlecleucel die erste CAR chimeric antigen receptor-T Zelltherapie in den USA verfügbar.
Although both products are capable of targeting CD19 there are significant differences in their CAR design.
 Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development
Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development
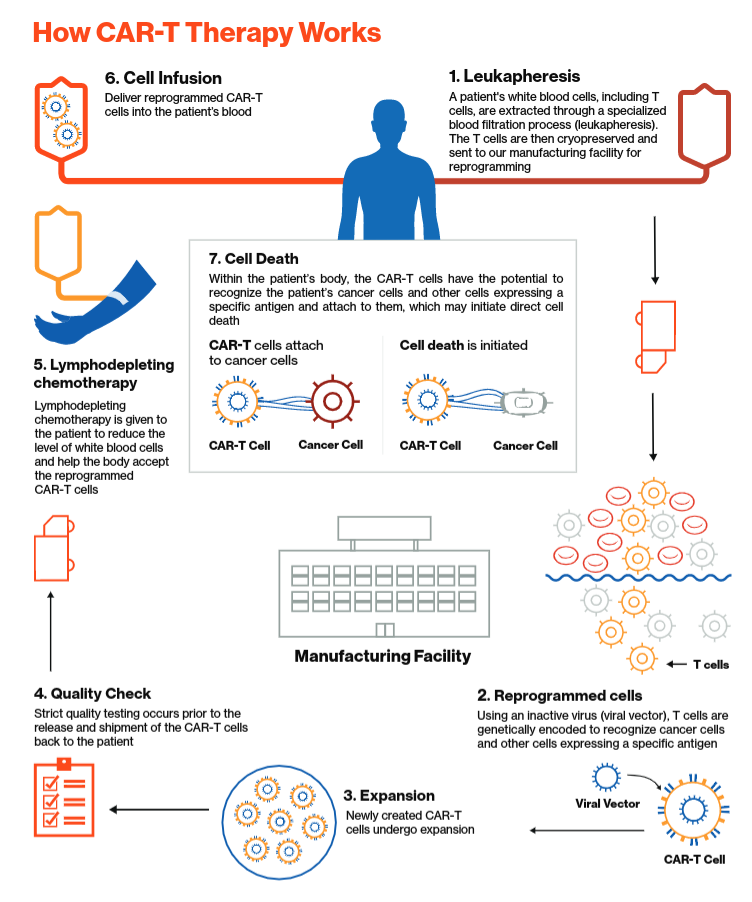 The Fda Approves A Second Car T Therapy Cheaper Than Novartis
The Fda Approves A Second Car T Therapy Cheaper Than Novartis
 Car T Mechanism Of Action Kymriah Tisagenlecleucel
Car T Mechanism Of Action Kymriah Tisagenlecleucel
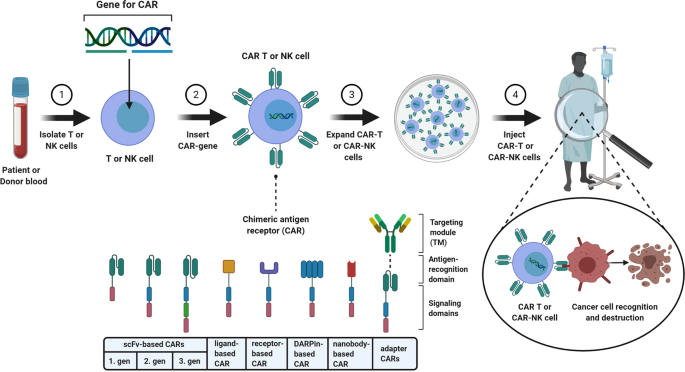 Current Status And Perspective Of Car T And Car Nk Cell Therapy Trials In Germany Gene Therapy
Current Status And Perspective Of Car T And Car Nk Cell Therapy Trials In Germany Gene Therapy
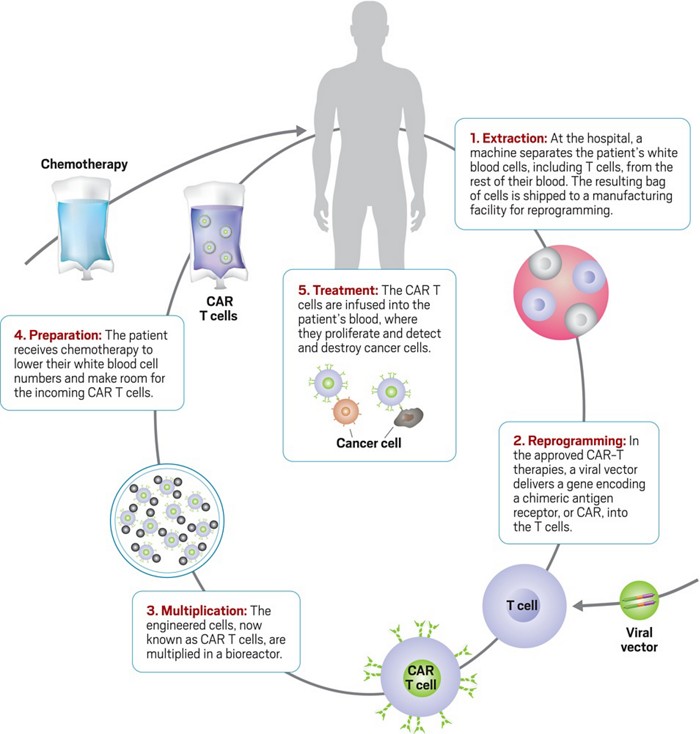 Manufacturing Challenge Leads To Death In Car T Trial
Manufacturing Challenge Leads To Death In Car T Trial
 Frontiers The Advent Of Car T Cell Therapy For Lymphoproliferative Neoplasms Integrating Research Into Clinical Practice Immunology
Frontiers The Advent Of Car T Cell Therapy For Lymphoproliferative Neoplasms Integrating Research Into Clinical Practice Immunology
 Car T Zell Therapie Novartis Zieht Patent Von Kymriah Zuruck
Car T Zell Therapie Novartis Zieht Patent Von Kymriah Zuruck
 Chasing Cars To Treat Cancer The Dawn Of New Therapies Candriam De
Chasing Cars To Treat Cancer The Dawn Of New Therapies Candriam De
Yescarta Vs Kymriah Which Car T Product Is Better Drugood
How Many Patients Car T Cell Therapy Can Treat
 Erstattungsmodell Fur Die Car T Zelltherapie
Erstattungsmodell Fur Die Car T Zelltherapie
 Car T Novartis Prices Ctl019 At Us 475 000 European Biotechnology
Car T Novartis Prices Ctl019 At Us 475 000 European Biotechnology
 Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
Fda Approves First Car T Cell Therapy The Evolution Of Car T Cell Therapy
 Car T Cell Therapies Current Limitations Future Opportunities
Car T Cell Therapies Current Limitations Future Opportunities
Comments
Post a Comment