Featured
- Get link
- X
- Other Apps
Olaparib Prescribing Information
Contraceptives may be reduced if co-administered with olaparib see also sections44and46. HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYNPARZA safely and effectively.
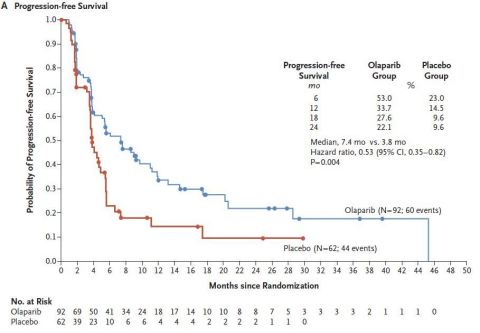
 Olaparib Tablets As Maintenance Therapy In Patients With Platinum Sensitive Relapsed Ovarian Cancer And A Brca1 2 Mutation Solo2 Engot Ov21 A Final Analysis Of A Double Blind Randomised Placebo Controlled Phase 3 Trial The Lancet Oncology
Olaparib Tablets As Maintenance Therapy In Patients With Platinum Sensitive Relapsed Ovarian Cancer And A Brca1 2 Mutation Solo2 Engot Ov21 A Final Analysis Of A Double Blind Randomised Placebo Controlled Phase 3 Trial The Lancet Oncology
See full prescribing information for LYNPARZA.
Olaparib prescribing information. Wenn Sie stillen siehe weiter unten im Abschnitt 2 für weitere Informationen. Rubraca rucaparib prescribing information. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION.
See full prescribing information for LYNPARZA. N Engl J Med. Variation assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted.
LYNPARZA olaparib prescribing information. Golan T Hammel P Reni M et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer.
Talzenna talazoparib full Prescribing Information. GBRCAtesting helps inform treatment choices including olaparib LYNPARZA a targeted maintenance therapy for patients with metastatic pancreatic cancer and gBRCAm whose disease has not progressed on 16 weeks of first-lineplatinum-based chemotherapy12 Test your patients with the FDA-approved BRACAnalysis CDxfrom Myriad Genetics. LYNPARZA is a prescription medicine used to treat adults who have.
26 72 123 CYP3A Inducers. If you are in the US and would like additional information regarding AstraZeneca drugs please contact the Information Center at AstraZeneca at 800-236-9933 800-236-9933. LYNPARZA olaparib prescribing information.
Wenn Sie nicht sicher sind sprechen Sie vor der Einnahme von. Wenn Sie allergisch gegen Olaparib oder einen der in Abschnitt 6 genannten sonstigen Bestandteile dieses Arzneimittels sind. Simvastatin pravastatin dabigatran digoxin andcolchicine.
Avoid concomitant use of strong or moderate CYP3A inducers as decreased efficacy can occur 73 123----- USE IN SPECIFIC POPULATIONS -----. 2018379262 49 5-2 50 5. HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYNPARZA safely and effectively.
Nehmen Sie Lynparza nicht ein wenn eine dieser Angaben auf Sie zutrifft. LYNPARZA olaparib prescribing information. In vitro olaparib inhibits the efflux transporter P-gp IC50 76µM therefore it cannot be excluded that olaparib may cause clinically relevant drug interactions with substrates of P-gp eg.
Click to learn more information. Full Prescribing Information. Advanced ovarian cancer fallopian tube cancer or primary peritoneal cancer with a certain type of inherited germline or acquired somatic abnormal BRCAgene.
Learn more about how LYNPARZA olaparib combined with bevacizumab may give women with advanced ovarian cancer more time without their cancer growing or returning. LYNPARZA Olaparib Tablets 1 NAME OF THE MEDICINE. AUSTRALIAN PRODUCT INFORMATION.
FDA-APPROVED FOR FIRST-LINE MAINTENANCE USE. Moore K Colombo N Scambia G et al. N Engl J Med.
Olaparib is a white to pale yellow crystalline powder which is very slightly soluble in aqueous solutions 010013 mgmL at 37C slightly soluble in ethanol 55 mgmL at 37C and has a pKa of. HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYNPARZA safely and effectively. Zejula niraparib prescribing information.
Der Wirkstoff in Lynparza Olaparib hemmt die Wirkung von als humane PolyADP-Ribose-Polymerase PARP bezeichneten Enzymen die im Rahmen der Zellteilung an der Reparatur beschädigter DNA in. Talzenna talazoparib prescribing information. Research Triangle Park NC.
Monday to Friday 8 AM to 8 PM ET excluding holidays. LYNPARZA olaparib prescribing information. Weitere Informationen zur Anwendung von Lynparza entnehmen Sie der Packungsbeilage oder wenden Sie sich an Ihren Arzt oder Apotheker.
See full prescribing information for LYNPARZA. Rubraca rucaparib package insert. Zejula niraparib prescribing information.
Pujade-Lauraine E Ledermann JA Selle F et al. Outside of these hours and on holidays an after-hours service is available to assist you with any urgent medical issues. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer.
For Healthcare Professionals.
Lynparza Full Prescribing Information Dosage Side Effects Mims Hong Kong
 Treatment For Advanced Ovarian Metastatic Breast Pancreatic And Prostate Cancers Lynparza Olaparib
Treatment For Advanced Ovarian Metastatic Breast Pancreatic And Prostate Cancers Lynparza Olaparib
Https Rxisk Org Medication Guides Lynparza Pdf
 Ndc 0310 0668 Lynparza Olaparib
Ndc 0310 0668 Lynparza Olaparib
 Lynparza Olaparib First Line Maintenance Therapy Nearly Doubled The Time Without Disease Progression Or Death In Phase 3 Polo Trial For Patients With Germline Brca Mutated Metastatic Pancreatic Cancer Biospace
Lynparza Olaparib First Line Maintenance Therapy Nearly Doubled The Time Without Disease Progression Or Death In Phase 3 Polo Trial For Patients With Germline Brca Mutated Metastatic Pancreatic Cancer Biospace
 Pdf Olaparib As Maintenance Treatment For Patients With Platinum Sensitive Relapsed Ovarian Cancer
Pdf Olaparib As Maintenance Treatment For Patients With Platinum Sensitive Relapsed Ovarian Cancer
 Lynparza Olaparib Tablet Film Coated
Lynparza Olaparib Tablet Film Coated
 Combination Therapy Lynparza Olaparib Bevacizumab
Combination Therapy Lynparza Olaparib Bevacizumab
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2017 208558s000lbl Pdf
 Pdf Olaparib As Maintenance Treatment For Patients With Platinum Sensitive Relapsed Ovarian Cancer
Pdf Olaparib As Maintenance Treatment For Patients With Platinum Sensitive Relapsed Ovarian Cancer
 Lynparza Olaparib Efficacy For Gbrcam Her2 Negative Metastatic Breast Cancer
Lynparza Olaparib Efficacy For Gbrcam Her2 Negative Metastatic Breast Cancer
 Efficacy Of Lynparza Olaparib For Maintenance Of Advanced Ovarian Cancer
Efficacy Of Lynparza Olaparib For Maintenance Of Advanced Ovarian Cancer
 Buy Lynparza Olaparib Price Costs Thesocialmedwork
Buy Lynparza Olaparib Price Costs Thesocialmedwork

Comments
Post a Comment