Featured
- Get link
- X
- Other Apps
Interferon Beta 1a Contraindications
One single-dose prefilled AVONEX syringe. 1996 RECENT MAJOR CHANGES Indications and Usage 1 072019 Dosage and Administration Important Administration Instructions 22 032020 Contraindications 4 032020 INDICATIONS AND USAGE AVONEX is for the treatment of relapsing forms of multiple sclerosis MS to.
 Congenital Anomalies Detected In Live Birth Pregnancies Exposed To Ifn Download Table
Congenital Anomalies Detected In Live Birth Pregnancies Exposed To Ifn Download Table
Dagegen ist Interferon beta.
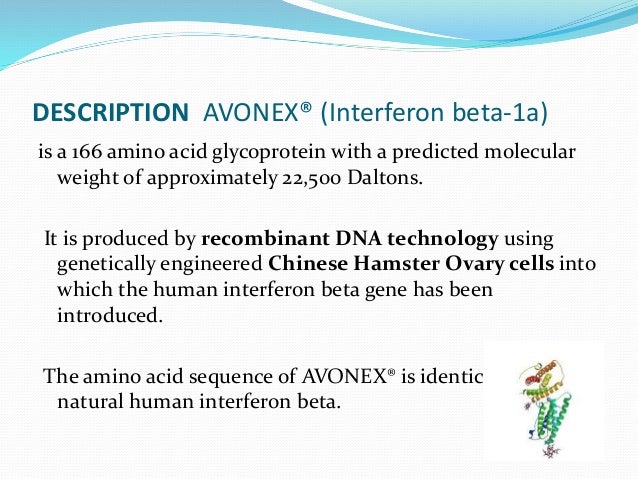
Interferon beta 1a contraindications. Adult Indications and Dosage FDA-Labeled Indications and Dosage Adult. Der Proteinanteil von Interferon beta-1a ist identisch mit dem des humanen physiologisch gebildeten Beta-Interferons das Glycosylierungsmuster ist sehr ähnlich. 163 Zeilen Interferon beta-1a is a form of recombinant human interferon used to.
One 23-gauge 1-inch needle. REBIF interferon beta-1a is formulated as a sterile solution in a prefilled syringe or REBIF Rebidose autoinjector intended for subcutaneous sc injection. Each 05 mL 05 cc of REBIF contains either 22 mcg or 44 mcg of interferon beta-1a 2 mg or 4 mg albumin human 273 mg mannitol 04 mg sodium acetate and water for injection.
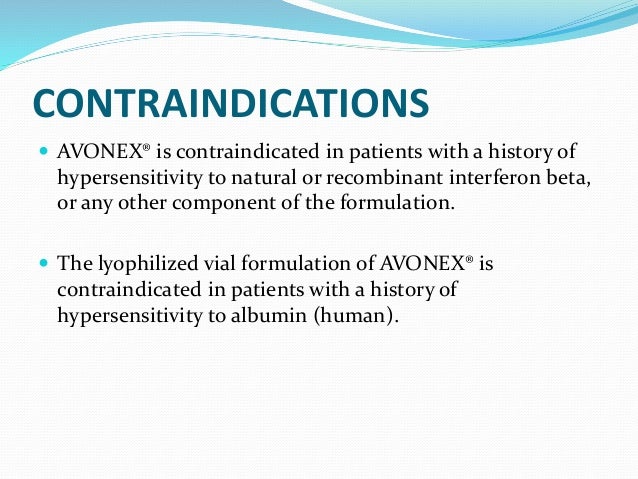
It is produced by mammalian cells while interferon beta-1b is produced in modified E. The formerly available lyophilized vial formulation of AVONEX is contraindicated in. Hypersensitivity to beta interferons albumin for albumin-containing formulations Cautions.
Interferon beta-1a should be used. Interferon beta-1a side effects. WebMD provides common contraindications for interferon beta-1a intramuscular.
AVONEX interferon beta-1a injection is a clear colorless solution in a single-dose prefilled glass syringe or a single-dose prefilled autoinjector for intramuscular injection available in the following packaging configurations. Interferon beta-1a is an immunologic adjuvant that is FDA approved for the treatment of multiple sclerosis. Get emergency medical help if you have signs of.
Subcutaneous administration of interferon beta-1a should not be substituted for. Der Proteinanteil von Interferon beta-1a ist identisch mit dem des humanen physiologisch gebildeten Beta-Interferons das Glycosylierungsmuster ist sehr ähnlich. Hepatic impairment pregnancy lactation depression may cause worsening suicidal.
Daher sollte das Medikament bei Eintritt einer Schwangerschaft abgesetzt werden. Alcoholism hepatic disease. Each 02 mL 02 cc of REBIF contains 88 mcg of interferon beta-1a.
CHO-Zellen produziert während zur Herstellung von Interferon beta-1b genetisch modifizierte Bakterien Escherichia coli verwendet werden. Es sind zwar bislang keine entwicklungsschä-digenden Wirkungen von Interferon-beta-Präparaten bekannt dennoch können negative Effekte nicht gänzlich ausgeschlossen werden. AVONEX interferon beta-1a injection for intramuscular injection Initial US.
It is not known if interferon beta-1a is excreted in breast milk. Angina cardiac arrhythmias cardiac disease heart failure myocardial infarction. Recombinant interferon beta or any other component of the formulationsee Warnings and Precautions 53.
CHO-Zellen produziert während zur Herstellung von Interferon beta-1b genetisch modifizierte Bakterien Escherichia coli verwendet werden. Find out what health conditions may be a health risk when taken with interferon beta-1a intramuscular. Common adverse reactions include flu-like symptoms including chills fever myalgia and asthenia.
CONTRAINDICATIONS PRECAUTIONS Subcutaneous administration. Interferon beta-1a also interferon beta 1-alpha is a cytokine in the interferon family used to treat multiple sclerosis MS. Interferon beta-1a is classified as FDA pregnancy risk category C animal studies show an adverse effect on the fetus.
Dagegen ist Interferon beta. Interferon beta-1a wird mithilfe von Säugetierzellen Ovarialzellen des chinesischen Hamsters. As many drugs enter breast milk and can potentially cause harm to the nursing infant interferon beta-1a should be used cautiously in nursing mothers.
Während der Schwangerschaft und Stillzeit sollte Interferon-beta nicht angewendet werden. Interferon beta-1a wird mithilfe von Säugetierzellen Ovarialzellen des chinesischen Hamsters. Some claims have been made that Interferons produce about an 1838 reduction in the rate of MS relapses.
 Interferon Beta 1b Contraindications
Interferon Beta 1b Contraindications
 Treatment Algorithm For Non Responders To Interferon Beta Or Glatiramer Download Scientific Diagram
Treatment Algorithm For Non Responders To Interferon Beta Or Glatiramer Download Scientific Diagram
Https Www Merckneurology Com Content Dam Web Healthcare Biopharma Neurology Merckneurology Docs Rebiff Pi Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2002 Ifnbbio111202lb Pdf
 Pdf Review Of The Clinical Evidence For Interferon B 1a Rebif In The Treatment Of Multiple Sclerosis
Pdf Review Of The Clinical Evidence For Interferon B 1a Rebif In The Treatment Of Multiple Sclerosis
Https Www Ema Europa Eu Documents Procedural Steps After Rebif Epar Procedural Steps Taken Scientific Information After Authorisation En Pdf
 The Innovative Development In Interferon Beta Treatments Of Relapsing Remitting Multiple Sclerosis Madsen 2017 Brain And Behavior Wiley Online Library
The Innovative Development In Interferon Beta Treatments Of Relapsing Remitting Multiple Sclerosis Madsen 2017 Brain And Behavior Wiley Online Library
 Pdf A Prospective Study Comparing Injectable Interferon Beta 1a Rebif 22 44 Avonex 30 And 1b Betaseron 250 Injection Site Reaction In Multiple Sclerosis Patients
Pdf A Prospective Study Comparing Injectable Interferon Beta 1a Rebif 22 44 Avonex 30 And 1b Betaseron 250 Injection Site Reaction In Multiple Sclerosis Patients
 Avonex Interferon Beta 1a Kit Avonex Pen Interferon Beta 1a Injection Solution Avonex Interferon Beta 1a Injection Solution
Avonex Interferon Beta 1a Kit Avonex Pen Interferon Beta 1a Injection Solution Avonex Interferon Beta 1a Injection Solution
 Interferon Beta 1a Serious Side Effects
Interferon Beta 1a Serious Side Effects
 Plegridy Peginterferon Beta 1a Dosage Uses Side Effects Emedz
Plegridy Peginterferon Beta 1a Dosage Uses Side Effects Emedz
 Methylprednisolone In Combination With Interferon Beta 1a For Relapsing Remitting Multiple Sclerosis Mecombin Study A Multicentre Double Blind Randomised Placebo Controlled Parallel Group Trial The Lancet Neurology
Methylprednisolone In Combination With Interferon Beta 1a For Relapsing Remitting Multiple Sclerosis Mecombin Study A Multicentre Double Blind Randomised Placebo Controlled Parallel Group Trial The Lancet Neurology
Comments
Post a Comment